
A Michigan judge has ruled that drug manufacturers are not protected by the Public Readiness and Emergency Preparedness (PREP) Act if the drug in question was contaminated; hence, the lawsuit filed by Ven Johnson Law on behalf of Dan Nowacki will proceed to trial.

The PREP Act shields manufacturers, administrators and distributors from liability claims resulting from harm caused by drugs or vaccines that received Emergency Use Authorization (EUA). The PREP Act, signed into law in December 2005 by then-President George W. Bush, overrides all federal and state vaccine safety laws in the case of an emergency declaration by HHS. Specifically, it exempts drug makers from lawsuits related to the manufacture, testing, development, distribution, administration and use of medical countermeasures against chemical, biological, radiological and nuclear agents of terrorism, epidemics, and pandemics.
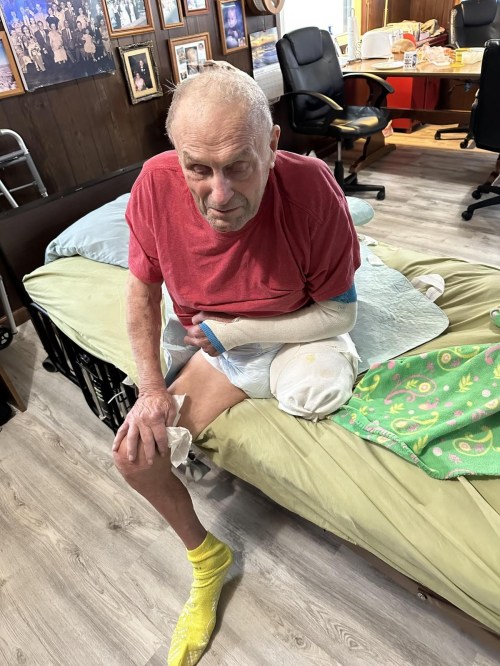
Remdesivir received Emergency Use Authorization (EUA) for use in COVID-19 patients. However, two lots containing 55,000 vials of the drug had to be recalled after it was found they were contaminated with glass particles. Nowacki received at least two of those contaminated doses after which he suffered two strokes and a leg amputation. The lawsuit was filed in Washtenaw County Circuit Court and names Gilead Sciences, Inc., the drug manufacturer, and St. Joseph Mercy Chelsea Hospital, Inc. as defendants.

This Michigan judge’s ruling in the Nowacki case establishes an important precedent, because it opens a door for those who were damaged by the ‘rona jab to sue Pfizer and/or Moderna. The basis for such a lawsuit would be a contaminant that researchers have found in all of the Moderna and Pfizer mRNA COVID-19 serums.

The first hint of this find appeared on April 10, 2023, in a paper entitled “Sequencing of bivalent Moderna and Pfizer mRNA vaccines reveals nanogram to microgram quantities of expression vector dsDNA per dose” that was published in OSF Preprints. The research team was headed up by Kevin McKernan, an expert in DNA and RNA sequencing who is best known for his role as a team leader in the Human Genome Project.In their paper, McKernan et al. reported finding dsDNA in both Moderna and Pfizer mRNA vaccines in excess of both the European Medicines Agency (EMA) 330ng/mg limit and the FDA 10ng/dose limit.
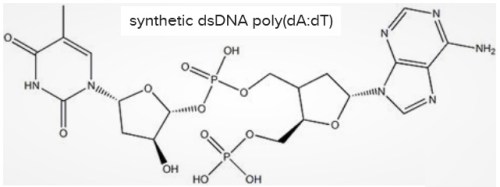
This is a problem, because dsDNA causes blood clotting. (Sound familiar?) On April 25, 2017, a paper entitled “Double-stranded DNA induces a prothrombotic phenotype in the vascular endothelium” was published in Nature. In it, researchers reported that the synthetic dsDNA (double-stranded DNA) known as “poly(dA:dT)” induced accelerated blood clotting in vitro in human micro-vascular endothelial cells – i.e., skin cells harvested from unborn humans (likely aborted fetal remains).
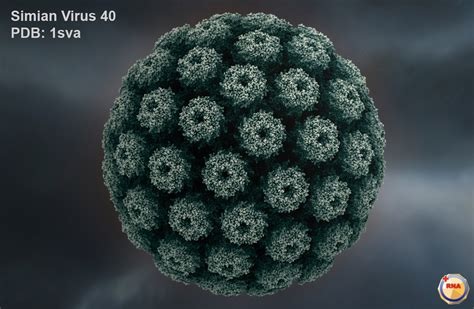
On October 19, 2023, Health Canada, the country’s public health authority, confirmed the presence of DNA contamination in Pfizer COVID-19 vaccines. The specific dsDNA they found is known as Simian Virus 40 (SV40), a DNA viral gene sequence. In April 2004, a paper entitled “Emergent Human Pathogen Simian Virus 40 and Its Role in Cancer” was published in the peer-reviewed medical journal Clinical Microbiology Reviews. It reported on the growing evidence that SV40 causes cancer. As with blood clotting, there has been a sharp increase in cancer since the COVID-19 “vaccines” were rolled out.

Health Canada also confirmed that Pfizer did not disclose the presence of SV40. Plasmid DNA is used in the manufacturing of mRNA vaccines, but it is supposed to be removed to a level below thresholds set by health regulatory agencies. In addition, Pfizer was required to disclose its use and presence in the serums to health regulatory agencies. They did not disclose, probably because the levels in their serums were and apparently continue to be many, many times higher than is considered safe.

On October 23, 2023, Steve Kirsch wrote, “Legal opinions will vary of course, but I just got off the phone with a very high profile attorney in the COVID litigation space (who asked not to be quoted since he hasn’t extensively researched this yet) and asked the key question: “Do you have to tie your harm to the adulteration specifically, or does the adulteration remove the liability protection on the base product?” He said, as expected, the case is stronger if you can link the adulteration as causing your injuries, but there is still liability because you didn’t give informed consent to having an adulterated product injected and the PREP act only protects the unadulterated product that you thought you were getting. Bottom line: if you can tie the vaccine with your injury you have a case. You have an even better case if you can tie the adulteration to your injury, but that is NOT necessary.”

A list of 90 lawyers that you can contact if you want to sue the vaccine manufacturers @ https://airtable.com/appGIUGnttjzscdJF/shrAqtpTOTkoOgrbx/tblfVuObLpclbtF0W

CLICK https://rumble.com/v3r1pqf-vaccine-adulteration-wkevin-mckernan-byram-bridle-chris-martenson-steve-kir.html [1:52:09] to listen to Steve Kirsch interview experts about the situation.
- https://en.wikipedia.org/wiki/Public_Readiness_and_Emergency_Preparedness_Act
- https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
- Emergency Use Authorization (EUA) for remdesivir, an unapproved product @ https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/EUA%20Review%20Remdesivir_050120.pdf
- https://wwmt.com/news/local/michigan-judge-denies-drug-manufacturers-immunity-in-case-of-contaminated-covid-19-medication
- https://www.fox2detroit.com/news/lawsuit-claims-metro-detroit-man-was-given-contaminated-covid-medicine-with-glass-shards
- https://twitter.com/stkirsch/status/1715820838564593745
- Sequencing of bivalent Moderna and Pfizer mRNA vaccines reveals nanogram to microgram quantities of expression vector dsDNA per dose @ https://osf.io/b9t7m/
- Kevin McKernan @ https://medicinalgenomics.com/team/kevin-mckernan/
- https://worldcouncilforhealth.org/multimedia/kevin-mckernan-plasmid-mrna-vaccines/
- Double-stranded DNA induces a prothrombotic phenotype in the vascular endothelium @ https://www.nature.com/articles/s41598-017-01148-x
- Order Poly(dA:dT) from Invivo Gen @ https://www.invivogen.com/poly-dadt-naked
- https://childrenshealthdefense.org/defender/canada-dna-contamination-pfizer-covid-vaccine/
- Primary Dermal Microvascular Endothelial Cells; Normal, Human, Neonatal (fetal skin cells) are not currently available@ https://www.atcc.org/cell-products/primary-cells/endothelial-cells
- ‘Probably the Most Important Topic of Our Time’: DNA Contaminants in COVID Shots Can Trigger Cancer, Alter Human Genome, October 11, 2023 @ https://childrenshealthdefense.org/defender/covid-vaccines-dna-contamination/
- Emergent Human Pathogen Simian Virus 40 and Its Role in Cancer, July 2004, Clinical Microbiology Review@ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC452549/
- https://en.wikipedia.org/wiki/Clinical_Microbiology_Reviews
- https://en.wikipedia.org/wiki/SV40
- https://twitter.com/stkirsch/status/1716522963690979607







